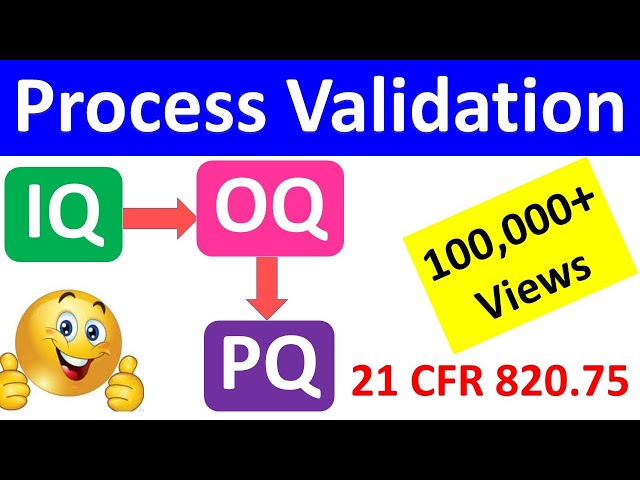
IQ OQ PQ, Process Validation, Equipment Validation
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.

Process Validation or Verification (Medical Device)?

Practical Process Validation
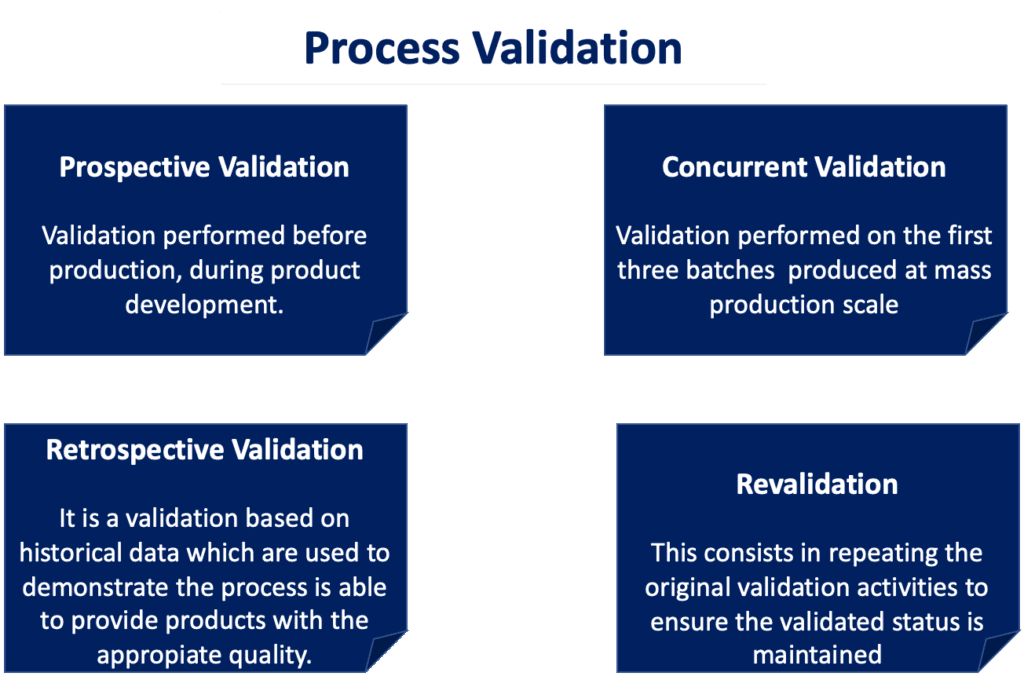
Process Validation for Medical Devices: Overview of FDA Requirements
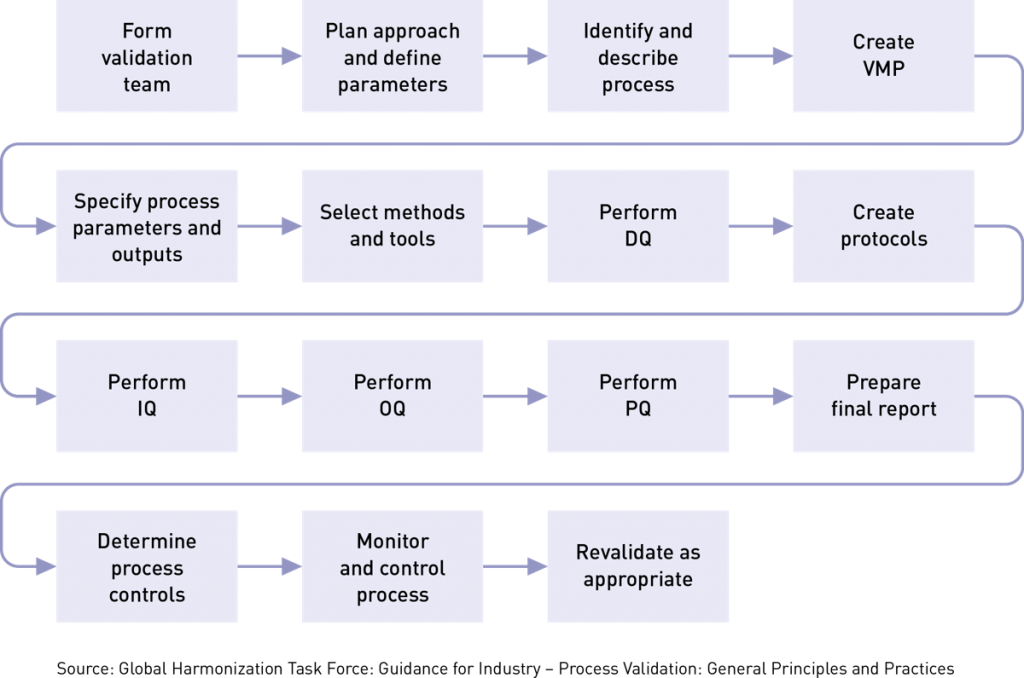
Overview of Medical Device Process Validation: IQ, OQ, and PQ – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
A Basic Guide to IQ, OQ, PQ in FDA-Regulated Industries
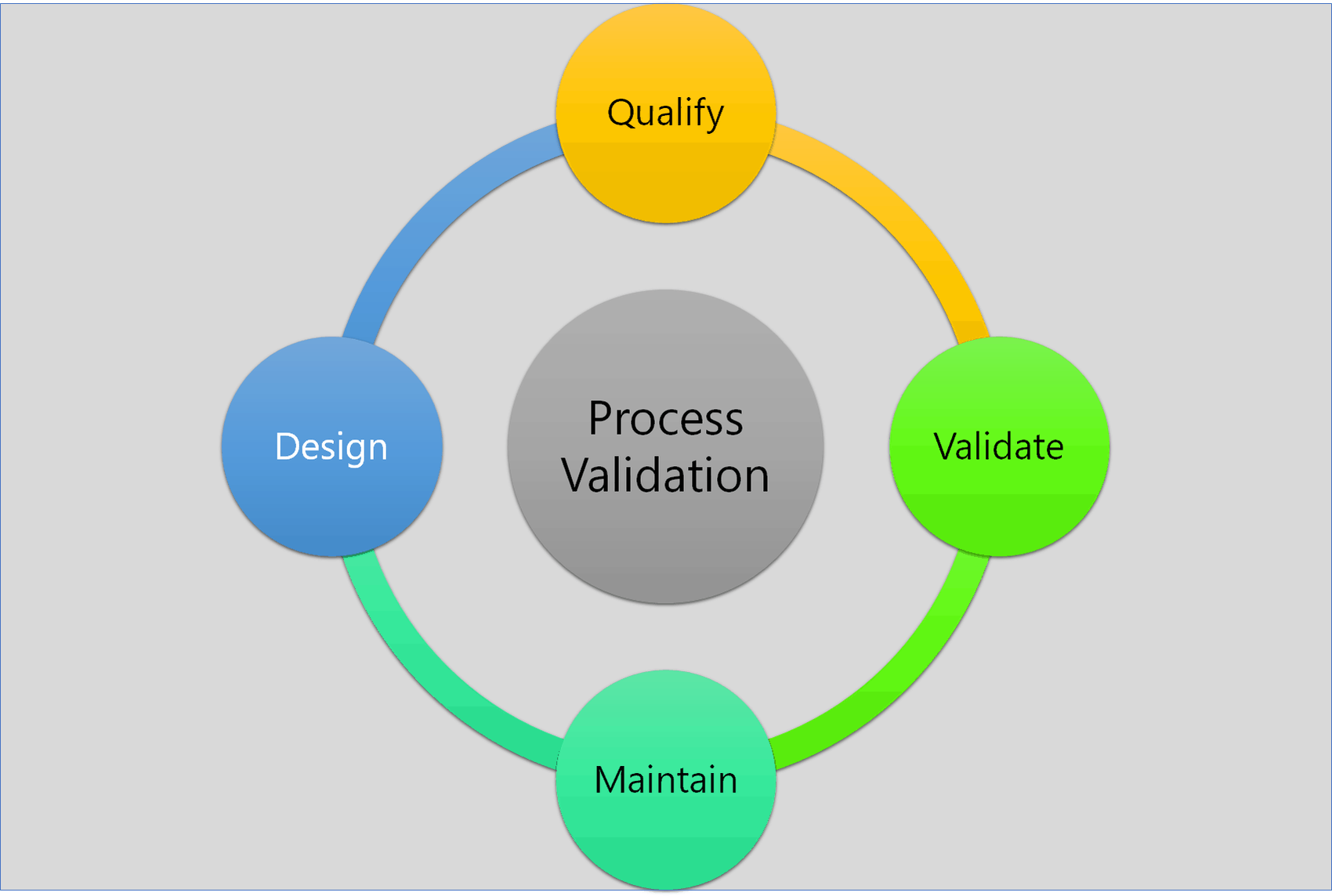
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
Validating a Pharmaceutical Research and Development Facility

Equipment Qualification - IQ, OQ, PQ Protocols : Compliance Training Webinar (Online Seminar)
Validation Protocols - Reports Procedure
Why did FDA change their Guideline on Process Validation? - ECA Academy

IQ OQ PQ Validation and Qualification Services

IQ/OQ/PQ in Equipment Verification and Validation - ANAB Blog

Process Validation or Verification (Medical Device)?

How to produce a superb combined IQ OQ PQ Protocol.
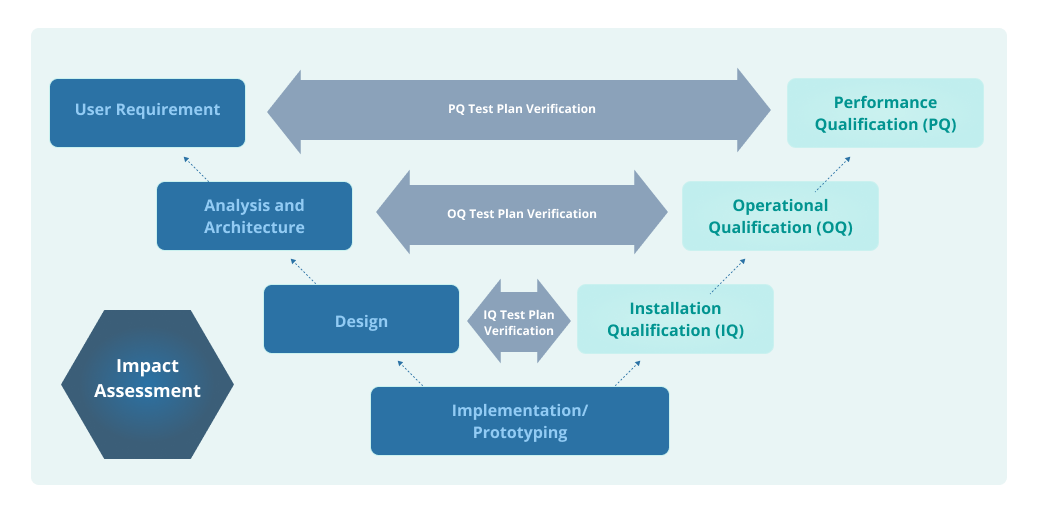
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries









